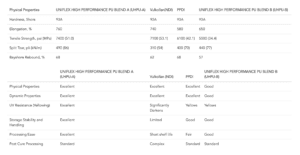
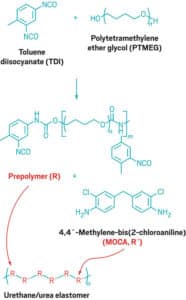
Molding Polyurethane: The Cast Urethane Process
Custom cast polyurethane products have become widely adopted throughout the manufacturing, food and beverage, mining, automotive, printing, and robotics industries. Their relative ease of production, low tooling costs, and longevity have made cast urethane parts a staple of industry and automation. But what is the process behind manufacturing custom molded urethane parts?
The Concept
It all starts with the concept. Generally, a customer will submit a CAD file. Or, some form of drawing file along with a brief description of the uses for the part, the environment where the usage of the part will take place, and quantity of the part they need. This is how the presentation of most inquiries happens, but it is not the only way. Sometimes customers do not have a design and need in-field engineering assistance to come up with a solution. In this case, an experienced engineer/estimator will drive or fly out to the company that is requesting the urethane product. And, works on the ground floor with the company in order to come up with a solution. Often, the quotation of the job can happen on-site. But, sometimes further engineering is a must in order to come up with an accurate estimate.
Quoting
Quoting is the second step in the process of molding polyurethane. Once the cast polyurethane parts manufacturer has the drawing of the part he will produce, estimating then thoroughly examines it and determines how much the part will cost. This step often requires involvement. And, it takes into account many things such as the best material to use for the part. Also, the cost of the material, the time it will take to make the tool, the time involved in setup processes, the time the job will spend in production. And, the time the part will spend in trimming and inspection.
Every little detail is important. Also, the estimator is often collaborating with all of these departments in order to come up with accurate time estimates so that the proper quotation of the job is possible. It is very important that all of these departments are working with incredible efficiency. This is in order to keep manufacturing costs low by saving time. This is because it ultimately provides the customer with a quality part at an economical price.
Tooling
Once the quotation of the job and the reception of the order happened, the first step in the production portion of the journey is tooling. Tooling is the process that results in the making of polyurethane molds and hobbs. Here, a machinist receives the drawing of the part in the form of an IGS file. The machinist is then able to take that design and come up with a CNC tool path. The tool path will make a mold for the part that when filled with urethane will form the part with extreme precision (usually within 0.005”). This can often be a very intricate process as urethane parts become more complex.
When complex polyurethane molds are made, they often have to be built in many pieces that come together in sync to form the cavity that will form the finished product. An alternative option to a cavity-cut mold, as mentioned previously, is a hobb. A hobb is an aluminum replica of the part that has been modified for the purposes of making a urethane mold. A hobb is designed to mimic the part so that when urethane is cast around it. And then its removal from the urethane ensues after a cure cycle. The result is a cavity that will produce the part when filled with urethane.
Production
After the production of the mold or hobb finishes, it then enters the production room. The production room is where molding polyurethane parts in mass takes place. Here, urethane is formulated using different prepolymers, curatives, and ratios to create formulations that are specific to the particular job. These specially formulated mixtures are generally processed in automated dispensers, centrifugal mixers, or hand-batched depending on the job size and requirements. After the material is processed it is then poured or dispensed into the polyurethane molds and cured in an oven, on a hot table, or in a heated hydraulic press (for compression molded parts). Most parts cure within a few hours because they are run with a more standard polyurethane formulation, but other formulations, such as higher-end Vulkollan substitutes, can take all day.
Trimming and Secondary Machining
In fact, trimming comes after production when the newly cast polyurethane parts have fully cured. Trimming is just removing all of the “flash” from urethane parts that have just come out of production. This is essentially just a clean-up operation where shaving off excess, leftover urethane from the molding process happens, creating the finished product.
Polyurethane parts may also need secondary machining operations, depending on the final use. For this, the urethane parts are moved back into the machine shop. There, they are turned, milled, 3D’d, or grinded (depending on the type of project and tolerances).
Inspection
Once the urethane part has made it through every operation, it still has one more step in the journey before its shipment happens: inspection. Inspection consists of checking the durometer of the finished polyurethane parts. Then, checking critical dimensions, checking color to make sure the ratio was correct for every mix, checking for porosity. And, checking for overall cleanliness. Here at Uniflex, we will not ship anything unless it went through a thorough inspection. If a part does not pass any step in the inspection process, we throw it out. And, we manufacture a new one.
Shipping
The final step in the process of molding polyurethane is shipping. Once the engineering of the polyurethane parts finishes, molds have been made, production has taken place, trimming is complete, and inspection is passed, the parts can finally be shipped out the door to the customer.
Conclusion
As you can see, the process of molding polyurethane requires involvement. There are a lot of steps along the journey from concept to finished part. So, a very professional and well-trained crew is essential for keeping things working efficiently. Without a dedicated staff and years of experience, it is very difficult to produce quality urethane products with speed, efficiency, and ultimately an economical price.
If you have any questions about polyurethane products, molding polyurethane, or the manufacturing process, do not hesitate to give us a call at (248) 486-6000 or send us a message. We have an experienced staff that is always willing to lend support!