Not All Urethane Is Equal: Quick Urethane Chemistry
Urethane has gained its reputation as one of the toughest and most versatile polymers known to date. From the mining, automotive, and oil industries all the way over to the skateboard wheel and gym equipment manufacturers; urethane has proven itself as a reliable material for many decades. What exactly is urethane, though, and are all urethanes the same?
For those of you that would like a quick answer to this question, it is a definite no. Not all urethanes are the same, and I am not just talking about different hardness, shapes, or colors either. I am talking about on a molecular level. Urethane’s physical properties such as resiliency, tensile strength, rebound, and modulus are all subject to change based on the chemistry of the urethane that is in usage. The important thing to realize is that there are many different “grades” of urethane; polyester resins, PPG and PTMEG polyether’s, as well as MDI based systems.
Now I know this is somewhat of a foreign language to most. However, we’ll break this down a bit and try to get some clarity.
MDI’s and TDI’s
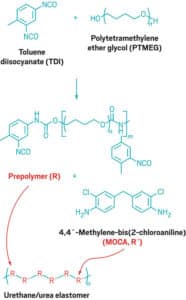
MDI’s and TDI’s are what are known as diisocyanates or the monomers of polyurethanes. MDI stands for methylene diphenyl diisocyanate and TDI stands for toluene diisocyanate. Both MDI and TDI diisocyanates combined makeup 90% of the overall diisocyanate market. And, their major use is in the manufacture of polyurethanes (1). In order to make a polyurethane prepolymer, one has to react to a diisocyanate (MDI or TDI) with a polyol. We could refer to a polyol as the “backbone” of a polyurethane molecule and it is where most of a urethane’s physical properties take root. For example, let’s come back to a type of urethane we mentioned earlier; a PTMEG polyether. PTMEG (polytetramethylene ether glycol) is a polyol or backbone whose usage is common in high-performance urethane systems. To produce a PTMEG polyether prepolymer, PTMEG needs to react with TDI’s (see reaction below).
Now, realize that this reaction of a diisocyanate with a polyol produces what is a called a prepolymer. A prepolymer is essentially just a syrupy or waxy resin that has to react with a curing agent in order to produce a urethane elastomer (urethane elastomers produce urethane parts).
Curing Agents
There are many different types of curing agents that can used to produce urethane elastomers. These curing agents can also modify the physical properties of the urethane. Different curing agents can go into mix in different ratios with prepolymers to achieve different hardness ranges and different physical properties such as tensile strength and rebound.
A very common curing agent whose usage is wide across the polyurethane industry is 4,4’-methylene-bis(2-chloroaniline); commonly known as MOCA (you can see it reacting with a prepolymer in picture above). This compound reacts with a prepolymer such a PTMEG polyether to create a high-performance polyurethane elastomer which is excellent for dynamic applications such as rollers.
Conclusion
I know is this is a big gulp of chemistry. But, the important takeaway from this very brief urethane chemistry lesson is to be careful when selecting who manufactures your polyurethane parts because not all urethane is equal. Be prepared to ask questions about the materials (prepolymer and curing agents) that are being used to manufacture your parts.
Realize that polyurethanes are a family of compounds and they all have different attributes that can be favorable or not favorable when costs are being cut (some materials are designed to be cheap). Finally, if you’re uncertain just ask. If you’re unsure about anything, give us a call at 248-486-6000 or email us for any questions you may have regarding urethane elastomer systems or urethane parts in general.
I also highly recommend checking out this article if you are interested in a more in-depth lesson on MDI and TDI based systems: https://catalogimages.wiley.com/images/db/pdf/0471958123.01.pdf
References:
- MDI, TDI and the Polyurethane Industry. (n.d.). Retrieved from https://catalogimages.wiley.com/images/db/pdf/0471958123.01.pdf